PRESS RELEASE
Metcela and Japan Lifeline to enter into a business partnership in preparation for commercialization of heart failure cell therapy | Metcela Inc.
Metcela and Japan Lifeline to enter into a business partnership in preparation for commercialization of heart failure cell therapy
Aiming for early product launch by strengthening cooperation between the two companies in product development and supply
Metcela Inc. (Co-CEO Takahiro Iwamiya, Kenichi Nogami) and Japan Lifeline Co., Ltd. (President and CEO Keisuke Suzuki)announced today that they have signed a business partnership agreement, strengthening the cooperation between the two companies in the development of cell therapy for heart failure treatment. Under the alliance, Japan Lifeline and Metcela aim to accelerate the completion of clinical trials and commercialization of MTC001, which consists of the precision injection catheter and the cell product.
Image of cooperation for development and launch of MTC001
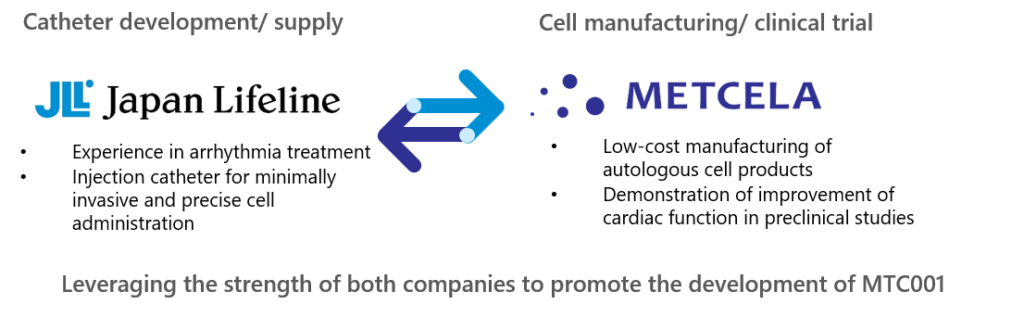
Prospective heart failure treatment that can be achieved through this business partnership
MTC001 is a regenerative medicine-based combination product, composed of special cardiac fibroblast (VCF), developed by Metcela, and an injection catheter, developed jointly with Japan Lifeline. The product is in preparation for the initiation of a clinical trial in early 2021.
Through basic and pre-clinical studies, Metcela has shown that VCF promotes cardiomyocyte proliferation in the diseased areas of the heart and thereby stimulates the regeneration of the heart tissue. For MTC001, Metcela plans to use an autologous cell product manufactured from the patients’ own biopsied cardiac tissue. Unlike allogeneic cell products, autologous cell products can evade immunological rejection after transplantation and achieve prolonged therapeutic efficacy while allowing for repeated administration of the product.
Metcela and Japan Lifeline have conducted several VCF administration studies in the porcine heart failure model, using the newly developed catheter, and confirmed the improvement in cardiac function. In these injection studies, it was also confirmed that repeat administrations of VCF by catheter have further enhanced the cardiac function. The two companies together see high business affinity and incredible market potential in autologous cell products, which can minimize the risk of immunological rejection, as well as injection catheters, which are minimally invasive and optimized for cell delivery.
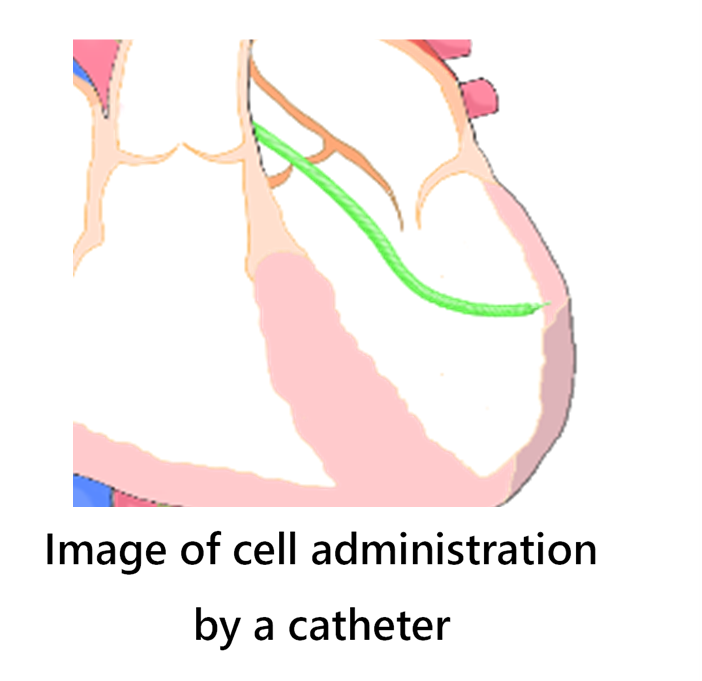
The catheter technology of Japan Lifeline enables to the precise targeting for cell administration
The administration of VCF is carried out transendocardially by injecting the cells via a catheter from the endocardium into the myocardial tissue. By utilizing a new catheter, which was specifically designed and developed for VCF, the MTC001 treatment is expected to lower the hurdles in the cell therapy field. Catheter-based cell administration eliminates the need for thoracotomy, which has been a major issue for many cell products for heart failure. It has also been confirmed in past studies that accurate and reliable administration of cells to targeted sites is imperative for achieving excellent therapeutic efficacy. Japan Lifeline holds extensive experience and technical expertise in developing and manufacturing catheters for diagnosing and treating arrhythmias. Fully utilizing these strengths, the development of a catheter with excellent operability equipped with a highly functional injection needle contribute to realizing reliable and safe cell injection.
Japan Lifeline aims to expand into new fields by leveraging its own expertise in arrhythmia treatment
Japan Lifeline handles cardiac pacemakers and catheters for arrhythmia treatment and has approximately 40 years of experience and a proven track record in the field of cardiovascular medicine. By combining VCF, an innovative fibroblast therapy, with advanced catheter technology, both companies are expecting to realize a combinational cell therapy product that has never existed before. As a medical device manufacturer, Japan Lifeline can utilize the opportunity to expand into a new area of regenerative medicine.
Comments from each company
Keisuke Suzuki (President and CEO of Japan Lifeline) comments:
Building a cooperative relationship with Metcela, who has advanced knowledge in cell therapy, opens new possibilities for utilizing the catheter technology we have cultivated over the years in arrhythmia treatment, for regenerative medicine products. We have already been preparing for commercialization through joint research and development as well as capital investment to Metcela. With this business partnership, we will further accelerate product development and commercialization.
Kenichi Nogami (Co-founder, Co-CEO of Metcela) comments:
Through the new business alliance with Japan Lifeline, VCF can be administered via catheter, which reduces the burden on patients and allows for repeated administration. Being a major shareholder of the Metcela, Japan Lifeline holds a solid track record in the development of cardiac catheters and has deep relationships with a wide range of medical institutions, and by maximizing our strengths and relationship with each other, we hope to accelerate the launch of MTC001 and global business expansion.
About Heart Failure
Heart failure is a complex condition in which the ability of the heart to pump blood decreases due to various causes. Majority of currently available treatment options only prevents the progress of the symptoms and preserve quality of life, and thus, is unable to fundamentally cure the irreversible ailment.
About VCF
Metcela discovered VCAM-1-positive Cardiac Fibroblast (VCF), a specific type of fibroblast that re-establishes favorable microenvironment within the damaged heart tissues. VCF can easily be cultured and is known to induce lymphangiogenesis and enhance proliferation of cardiomyocytes to regenerate the damaged tissues of the heart. Metcela’s intellectual property is covered by several patents (JP6241893 and JP6618066).
■Metcela Corporate Overview
| Headquarter: | Tsuruoka-shi, Yamagata |
| Research center: | Life Innovation Center 3-25-22 Tonomachi, Kawasaki-ku, Kawasaki-shi, Kanagawa |
| Representatives: | Co-CEOs Takahiro Iwamiya, Kenichi Nogami |
| Foundation: | March 9th 2016 |
| Website: | https://www.metcela.com/en/ |
| Business overview: | Research and development of regenerative medicine products for heart failure treatment |
■Japan Lifeline Corporate Overview
| Headquarter: | 2-2-20, Higashishinagawa, Shinagawa-ku, Tokyo |
| Representative: | President and CEO Keisuke Suzuki |
| Foundation: | February 6th, 1981 |
| Website: | https://www.japanlifeline.com/ |
| Business overview: | Import, development, production, and distribution of medical devices |
You may access the PDF version of our press release from the following link:
Metcela and Japan Lifeline to enter into a business partnership in preparation for commercialization of heart failure cell therapy
