PRESS RELEASE
Metcela eligible to receive up to $7 Million (1 Billion JPY) AMED Grant for Promoting the Development of Regenerative Medicine Product Using Cardiac Stem Cells | Metcela Inc.
Accelerate the Phase III clinical trial of cardiac stem cell-based product for pediatric congenital heart disease patients
Kanagawa, Japan – Metcela Inc. has been selected by the Japan Agency for Medical Research and Development (“AMED”) for the start-up type of the Cyclic Innovation for Clinical Empowerment (CiCLE) called ViCLE.

■About the selected project
The project will focus on the development of JRM-001, autologous cardiac stem cell-based product, for pediatric congenital heart disease patients and proceed with the Phase III validation study to confirm its efficacy and safety through collaboration with Okayama University. With this selection Metcela will be eligible to receive up to 1 billion JPY in contract funding for up to 5 years.
JRM-001 is subject to the Japanese Ministry of Health, Labor and Welfare’s SAKIGAKE Designation, and is expected to be launched as a breakthrough treatment for rare diseases in children.
■About JRM-001
Targeting single ventricle and its challenges
Single ventricle is a rare congenital heart disease in which the heart has only one functioning ventricle to pump blood, and it affects about 310 to 810 people per year in Japan[i]. Although series of anatomical repair surgery is performed as a standard of care and the postoperative survival rate has been improving in recent years, 90% of postoperative patients will develop cardiac dysfunction or heart failure by the age of 40. The 20-year survival rate is about 69%, making it a disease with a poor prognosis[ii]. With repeated surgical procedures starting in early childhood and the diseases’ impact on the growth and employment in adulthood, these patients often experience reduced quality of life. Despite these debilitating impacts, no drugs or regenerative medicine products that are indicated to have cardiac functional improvement have been approved to date.
Treatment concept
JRM-001 is a regenerative medical products that is mainly consisted of cardiac stem cells (CSCs). During standard of care surgery, a small piece cardiac tissue is biopsied and used as the starting material for JRM-001. The tissues are then transported to a cell processing facility and they are cultured and formulated. The final product is transported back to the medical facility and administered back into the patient’s heart during routine cardiac catheter examination (Figure).
As an autologous product, JRM-001 is characterized by minimal burden on patients and low risk of immune rejection.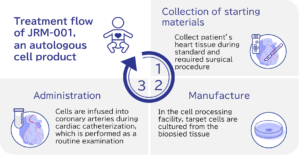
Development results to date
Metcela acquired Japan Regenerative Medicine Co., Ltd. (JRM) in April 2022 and succeeded the development of JRM-001. Research results suggesting the safety and efficacy of the treatment using CSCs for single ventricle have been established in the Phase I[iii] and Phase II[iv] clinical studies conducted by Okayama University. Based on these results, Phase III validation study was initiated.
■Comments from the development team
Dr. Shingo Kasahara
Professor and Director, Department of Cardiovascular Surgery, Okayama University
Director, Department of Pediatric Cardiovascular Surgery
The project selected focuses on the development of regenerative medicine product to treat a congenital heart disease called “single ventricle”.
The development of various surgical procedures has improved the prognosis for patients with single ventricle. However, the major issue is that some of the patients suffer from severe heart failure due to the loss of function of the cardiac muscle, a common characteristic of single ventricle. This severe heart failure also causes a chain reaction of multiple organ dysfunction and failure throughout the body.
Through two clinical studies conducted to date, Okayama University has confirmed the long-term efficacy of cardiac stem cells. With this project, we hope to complete the Phase 3 validation study together with Metcela and bring this innovative treatment to patients as soon as possible.
Kohtaro Toda, Vice President of Japan Regenerative Medicine Division, Metcela Inc.
Japan Regenerative Medicine Co., Ltd. was founded in 2013, and since then, we have consistently worked on the development of JRM-001 with a strong sense of mission to bring a new therapeutic product to children in need. Upon merger with Metcela, we have succeeded in resolving various technical issues in a short period of time.
We are strongly convinced that the practical application of this technology will lead to hopes for patients with single ventricle, for whom treatment options are limited.
■Outline of Accepted Project
- Project title: Development of a treatment for pediatric patients with congenital heart disease using cardiac stem cells
- R&D organization
Representative Organization: Metcela Inc.
Organization: Okayama University - Grant period and total contracted cost: up to 5 years, maximum 1 billion yen
- Reference: https://www.amed.go.jp/koubo/17/01/1701C_00002.html
■About Metcela
Metcela Inc., established in 2016, is a clinical-stage biotechnology startup pioneering the research and development of fibroblast and stem cell-based therapy for chronic diseases that currently have limited therapeutic options. MTC001 is a combination product of autologous cardiac cells (VCAM-1-positive Cardiac Fibroblast, VCF) and a novel catheter delivery system targeting chronic heart failure patients. MTC001 offers two major advantages over other cell therapies: (1) the therapeutic cells are autologous (patient-derived) and homologous (tissue-specific i.e. cardiac-derived), which is most suitable for the heart, as it is a highly immunogenic organ, and (2) the minimally invasive catheter system is equipped with a highly functional injection needle specifically designed for this therapy to achieve reliable and safe administration of the cells.
Website: https://www.metcela.com/en/
[i] Our estimate from the Japan Intractable Disease Information Center, description of the disease: https://www.nanbyou.or.jp/entry/4369
[ii] Japan Intractable Disease Information Center, overview, diagnostic criteria, etc.: https://www.nanbyou.or.jp/entry/4370
[iii] Ishigami et al. Circ Res. 2015 Feb 13;116(4):653-664
[iv] Ishigami et al. Circ Res. 2017 Mar 31;120(7):1162-1173, Sano et al. Circ Res. 2018 Mar 30;122(7):994-1005
