PIPELINES
Metcela's Commitment
Metcela is pioneering the future of medicine with a new business model that allows us to grow as a company while helping children suffering from illness. We aim to create a society where this approach becomes the standard.
The strong scientific evidence we gain from developing our pipeline for pediatric and rare diseases will drive our next phase of growth. Based on this robust, proprietary data, we aim to create new value and achieve better healthcare. We will acquire data that can be applied not just in Japan, but for patients worldwide.
JRM-001
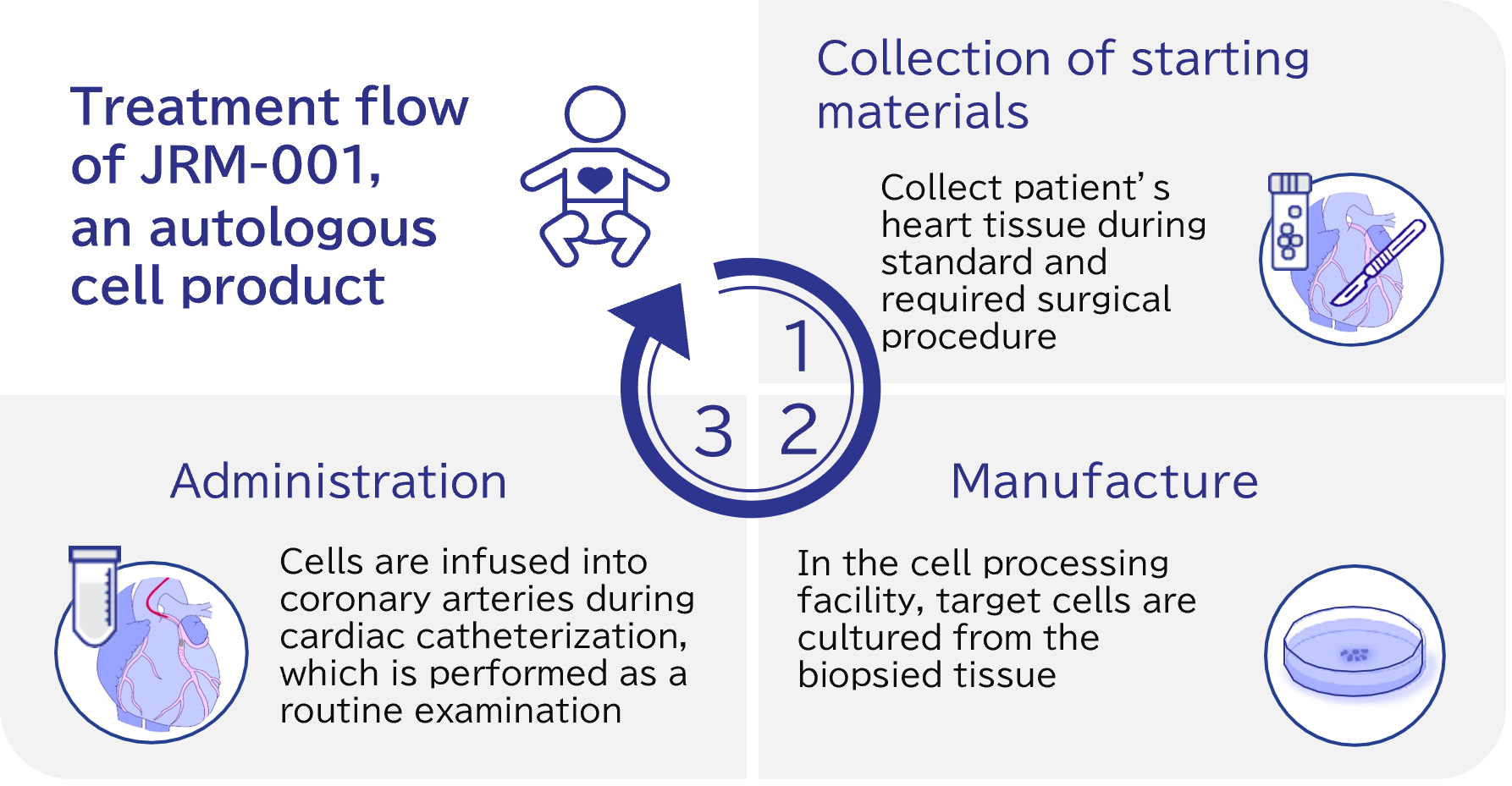
JRM-001 is an autologous cell therapy designed for sigle ventricle. It utilizes somatic stem cells naturally present in the heart, known as CSCs (Cardiac Stem Cells).
The core technology behind the "CSC-based pipeline," patented by Kyoto University, is centered on cells derived from the heart. Their natural compatibility with cardiac tissue holds great promise as an innovative therapeutic option for treating heart diseases.
Following the discovery, the safety and efficacy were reported by a research team at Okayama University Hospital in the world’s first clinical study using CSCs in pediatric patients with single-ventricle disease (Phase 1: TICAP , Phase 2: PERSEUS). These findings offer significant hope for new potential treatments for pediatric heart failure, particularly in Japan, where heart transplants are challenging to perform.
To advance development, we have obtained an exclusive license and technical guidance from Okayama University on manufacturing methods and clinical applications, and we are progressing toward practical implementation (jRCT1080223307, NCT02781922).
Since acquiring Japan Regenerative Medicine Co., Ltd. as a subsidiary, we have maintained the development of JRM-001, with the core team remaining onboard, including founder and development leader Kohtaro Toda (press release). The product has been selected for the Startup Type (ViCLE) of the Cyclic Innovation for Clinical Empowerment (CiCLE) by the Japan Agency for Medical Research and Development (AMED) (press release ). It is also designated under the Sakigake Designation System for Regenerative Medicine Product and as an Orphan Regenerative Medicine Product.
Single ventricle
Single ventricle refers to a condition where either the right or left ventricle of the heart does not function properly or is entirely absent. Classified as a rare disease, this condition often arises congenitally. With only one functioning ventricle, the heart is unable to pump blood effectively, leading to impaired blood flow to the body and lungs, and potentially resulting in insufficient oxygen supply.
In many cases, infants and young children undergo the Glenn procedure and Fontan-type surgery to establish a blood flow system called Fontan circulation, which supplies oxygen throughout the body as efficiently as possible. Patients with Fontan circulation require careful management and treatment, as significant risks of long-term complications and heart function deterioration remain, necessitating specialized medical monitoring. The patient’s prognosis largely depends on their specific condition and post-surgical progress.
Autologous cell therapy
Autologous cell therapy is a treatment that uses a patient’s own cells. Cells are collected from the patient’s body, cultured, processed, and then reintroduced to the patient to treat or support recovery from specific diseases. Autologous cell therapy is expected to be safer than treatments using cells from other donors (xenogeneic or allogeneic cell therapy) because it can avoid immune rejection.
MTC001
MTC001 is an autologous cell therapy designed for treating adult heart failure. It combines unique fibroblast (VCFs: VCAM-1-positive Cardiac Fibroblasts) that are particularly effective for cardiac tissue recovery with a dedicated catheter.
Administering the therapy via catheter is less invasive than open chest surgery, reducing both the physical and financial burden on patients while facilitating the product's introduction into the medical field.
Since 2021, MTC001 has been the subject of an investigator-initiated clinical trial. In 2024, both the trial and its development were halted due to significant challenges encountered in maintaining a stable supply of the investigational product. This cessation represents a strategic decision within our development pipeline and does not stem from any issues related to the efficacy or safety observed during the clinical trial.
Metcela remains dedicated to safeguarding the well-being of the patients who participated in this trial. In collaboration with the principal investigators, we plan to publish the data gathered from this trial in relevant academic forums and medical journals.
Applied research on VCFs is currently underway at LYMPHOGENiX LIMITED (London, UK) by our founding scientist and discoverer, Takahiro Iwamiya.
